KATA-ANA INVEST AB
is a company that develops charging and storage systems for energy.
Acquired Rights
For Investors

Kata-Ana invention
With Kata-Ana technology, it is estimated that the following can be achieved:
Electropositive metal ions are reduced to metal at normal temperature with analyzer and catalyst reactions with a pre-calculated and predetermined current. The current resistance of the solution and the required overvoltage have been taken into account. In battery technology, this means that the aluminum electrode gives off its energy and that it can be recharged enough times.
This is how you can manufacture batteries in all sizes with the following calculated performance values:
Energy density / volume: 2100 Wh / liter up to 8645 Wh/liter
Energy density / mass: 1330 Wh / kg up to 3340 Wh/kg
Charging times: 3000+
Minimum temperature: -37 Celsius, heated -50 Celsius
Maximum temperature: +70 Celsius to +120 Celsius
Operating time: 10-30 years
Dissolution rate: adjustable

Current development of batteries (accumulators)
Many difficulties on the development path.
In recent years, much work has been done to improve the capacity of rechargeable batteries, and this work has also yielded new results. New lead / acid, nickel / metal / hydride and lithium / ion batteries with a capacity of 100Wh / kg and 200Wh / kg have entered the market.
But the fact is that no radical innovation in terms of batteries has been presented for years. "New" batteries are without exception modifications made on old principles.
In the USA, the development consortium for electric cars, USABC, has set a goal of 300Wh / liter and 200Wh / kg. These are the minimum requirements for the electric car to be competitive with the traditional cars.
According to the latest news, USABC has created a nickel-metal hydride battery that produces approx. 100Wh / kg, but the manufacturing costs are many times greater than for lead / acid batteries.
None of the current batteries on the market will meet these requirements, as they have reached the extreme limit of their potential development. The basic problem is the weight of the batteries in today's market. A completely new solution is needed to reach new areas of use and expand the potential in today's market regarding batteries. The solution will be in the material aluminum.

Aluminum is a good solution for four reasons
a) It is available everywhere

History Aluminum batteries
The first notable attempt to build an aluminum battery was made by the US Philco Company in 1960. Philco's aluminum / air battery used aluminum with potassium hydroxide as the anode and air as the cathode. This battery stored energy 10 times more than the lead / acid battery and gave out 500 Wh / kg with a disc current of 1A / cm2.
The main setback was the corrosion that took place in the unloaded condition. The corrosion caused sediment in the aluminum hydroxide and explosive hydrogen gas formed. To avoid these problems, Philco added anti-corrosion inhibitors, and built a separate container where the electrolyte sediment was filtered. The battery had replaceable aluminum electrode plates.
A fresher experiment was made in 1985 by the DESPIC company, this time with salt electrolyte. By adding small amounts of tin, titanium, iridium or gallium, the corrosive properties were moved in a negative direction. DESPIC built its accumulator with wedge-shaped, replaceable anodes, which enabled mechanical charging through the use of salt water as electrolyte. The ALUPOWER company further developed it into a commercial version.
Some companies have used aluminum chloride, which at room temperature is a salt dissolved in water, and graphite electrodes that contain chlorine. This experiment was carried out in 1988 by Gifford and Palmisano and gave insignificant positive results due to the high resistance of graphite.
Equally significant were the results of Gileards and his working group, where they managed to save aluminum from organic solutions. The mechanisms behind these reactions are not properly understood even today.
During the years 1990-1995, Eltech Research (Fairport Harbor, Ohio) built a mechanically rechargeable aluminum battery for the PNGV program. It had 280 cells and it stored 190kWh with a peak power of 55kW. The battery weighed 195 kg and had a pumpable electrolyte system, where a separate filter removed the aluminum hydroxide sediment.
Applications similar to Kat-Ana technology are power capacitors used as emergency power plants, and electrolytic capacitors using aluminum electrodes and biological electrolytes.
It is especially noteworthy that power capacitors operate in extremely low temperatures. In Russia, 24V modules with an average of 150mm and a length of 60mm stored 20,000 Joules. They were used to start diesel engines at -40 degrees Celsius.
The significant thing in all attempts to take advantage of the energy of aluminum is that no one has been able to solve the recharging in other than mechanical ways (by replacing the used aluminum plate with a new one). When the right solution has not been found, the consequences have been such as sediment of aluminum hydroxide, too much resistance, corrosion problems, etc.
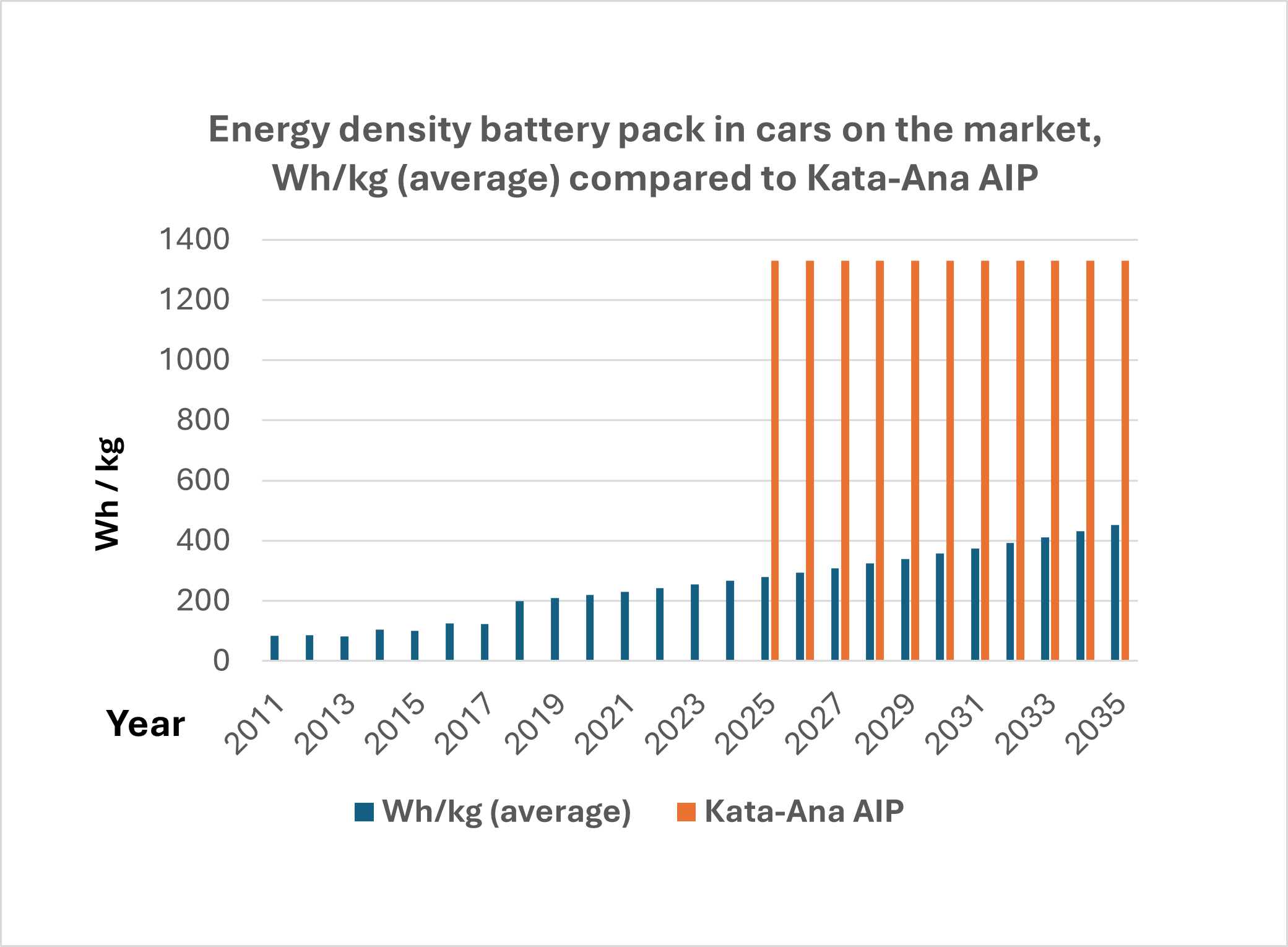
Energy density in electric car batteries - this is how it has developed until 2024. Forecast thereafter and comparison with Kata-Ana AIP batteries.
Manufacturers of electric car batteries have become increasingly better at squeezing more and more energy content per kilo of battery packs. The measure is called energy density and is measured in watt hours per kilo. Higher energy density means lighter cars and thus longer range, alternatively that you can hold more kilowatt hours in the same car. It is and will be a constant development.
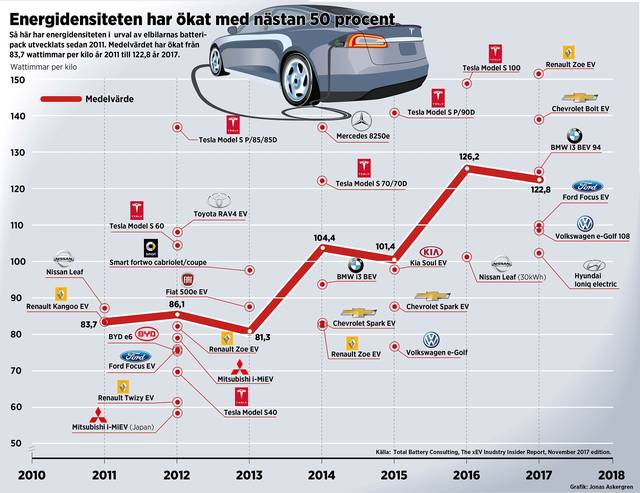
Energy density in electric car batteries - this is how it has developed
Manufacturers of electric car batteries have become increasingly better at squeezing more and more energy content per kilo of battery packs. Ny Teknik´s diagram shows the battery development of various car brands in the years 2010 - 2017.
Read more in the article Energy density in electric car batteries - how it has developed (nyteknik.se)

Comparison of material consumption and CO₂ emissions when manufacturing batteries.
Aluminum batteries are significantly more durable and environmentally friendly compared to lithium batteries. This advantage is crucial for large-scale battery production, where reduced CO₂ emissions and lower material requirements are essential for a sustainable future.

Kata-Ana wants to contribute to the development
We want Kata-Ana technology to be involved and contribute to the development of the electric vehicles, in a decisive way.
As a concrete example, one can take the world's first electric car that has achieved series production, General Motors EV1. There, the construction of the frame, the material and every smallest detail were built from start to finish, taking into account the conditions of the electric car's performance capability. The car's total weight without batteries is 816 kg. With batteries, the weight rises to 1,550 kg. As a power source, it has 26 lead / acid batteries of 53 Ah, which weigh 736 kg, ie almost half of the car's total weight. With a charge, the mileage with EV1 amounts to 145 km in highway driving and 115 km in city driving.
With Kata-Ana technology with an approx. 60kg power source with a volume of 40 liters, an energy capacity of 80 kWh is achieved. Installed in EV1 with a weight of 816kg, one would with a load achieve 870 km in highway driving and 690 km in city driving. The mileage is extended 6 times.